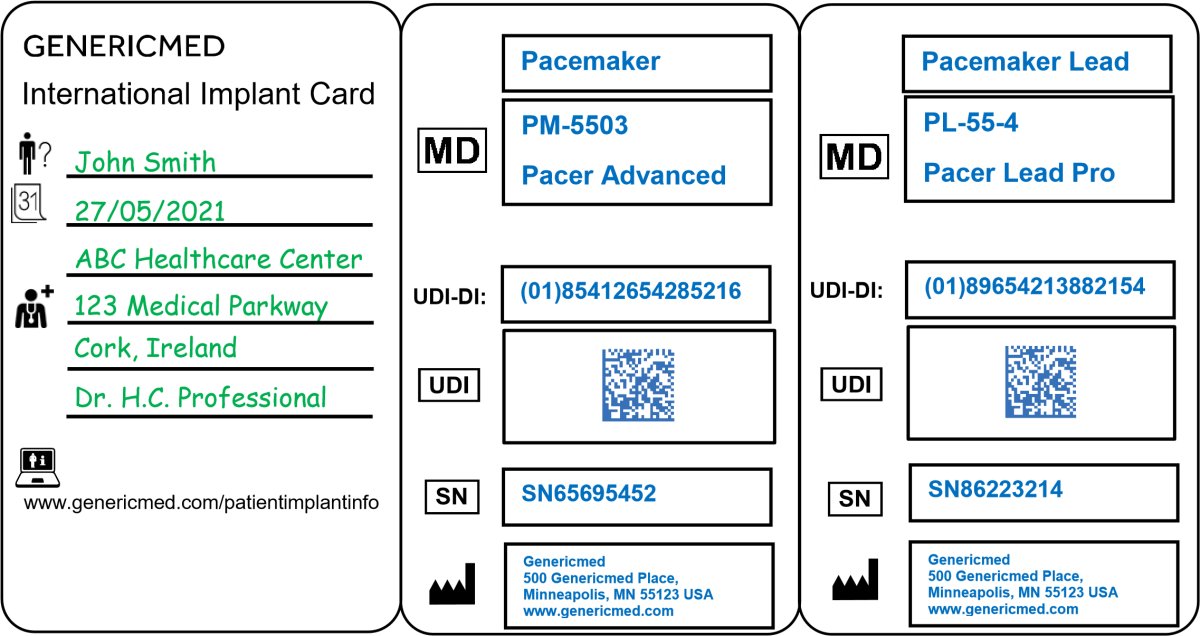
Guidance on Implant Card
This document describes the intended use, content and information to be provided by the manufacturer together on the IC and a definition of fields to be completed by the implanting healthcare institutions or healthcare providers according to national law in Member States.
Example of IC Leaflet