Entry into force of the IvDO!
The new Ordinance on In Vitro Diagnostic Medical Devices (IvDO) has entered into force on May 26, 2022 and the Ordinance English version is now available!
Check your obligations and the deadlines for your products.
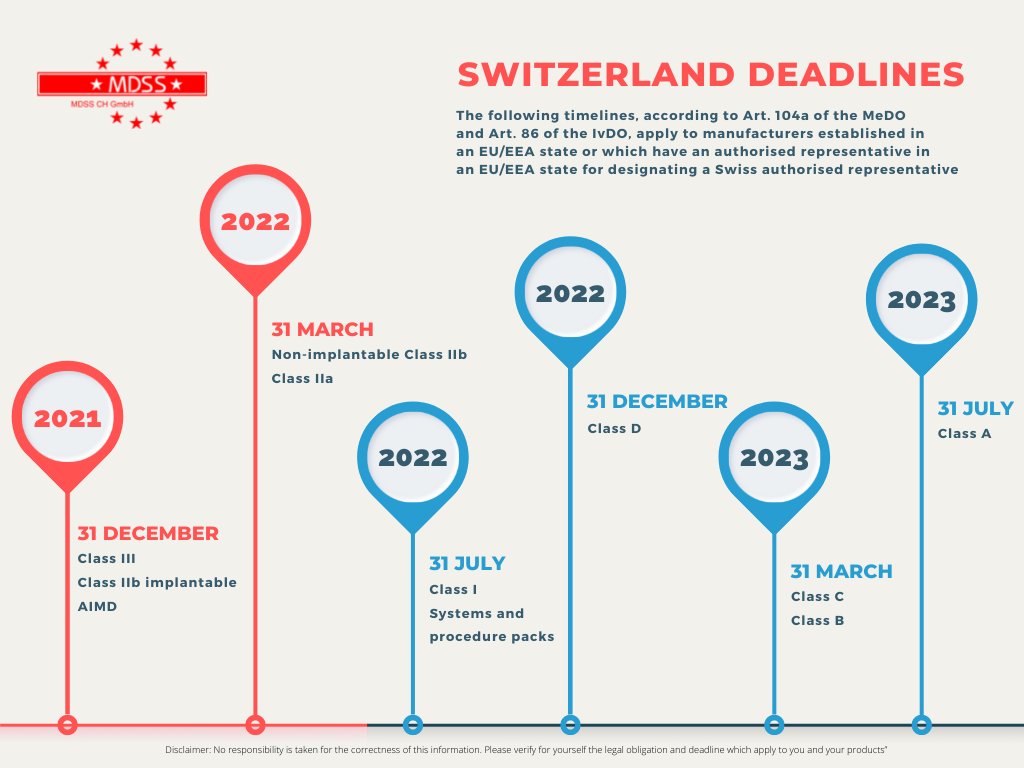
24/05/2022
IvDO adopted!
It is confirmed. Switzerland adopted the new Regulation on In-vitro Diagnostics (IvDO), which mainly refers to the IVDR.
The provisional text in German language is available here. Now we need to wait for the final version to be published in the Swiss Federal Council website.
Once it is published, check for yourself for your devices.
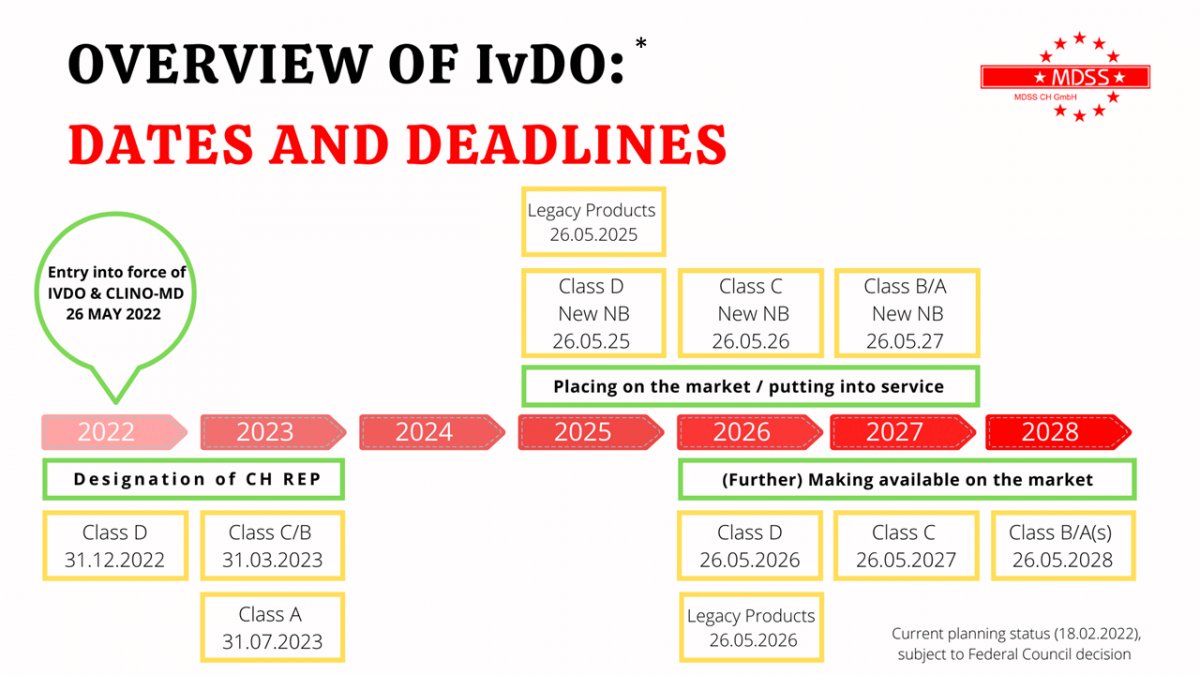
* based on the information from Swiss Medtech
Comment on youregulate.com...







