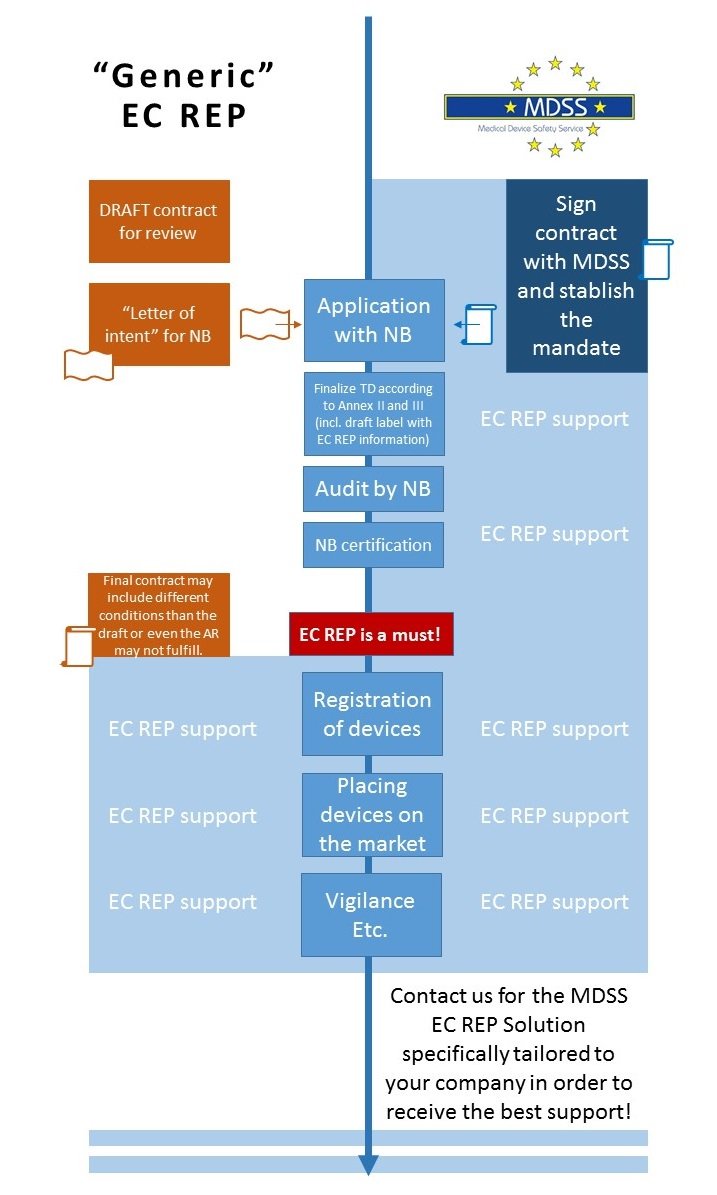
The importance of a European Authorized Representative (EC REP)?
The European Authorized Representative (EC REP) performs certain duties under the European Medical Devices Regulations and acts as the legal interface between the Medical Device manufacturer and the EU Authorities. Appointing MDSS as your EC REP will set your company on the forefront of European Regulatory Requirements. We meet the full EU criteria for CE marking and, most importantly, MDSS has the log-term experience in Regulatory Affairs to provide this demanding and `highly responsible´ Authorized Representative service!
When to designate the Authorized Representative?
We say: from the start! Ensure a smooth and efficient process!